Tutorial
These icons indicate there is something to be interacted with. Click it when you see it.
Tutorial
These icons indicate there is something to be interacted with. Click it when you see it.
Tutorial
These icons indicate there is something to be interacted with. Click it when you see it.
touchCONGRESS
 Our faculty interpret key data from the congress, complimented by an expert panel discussing what has been presented.
Close
Our faculty interpret key data from the congress, complimented by an expert panel discussing what has been presented.
Close
 Our faculty interpret key data from the congress, complimented by an expert panel discussing what has been presented.
Close
Our faculty interpret key data from the congress, complimented by an expert panel discussing what has been presented.
Close
Optimizing treatment of CML in the first-line setting and beyond: Insights from EHA 2024 and ESH-iCMLf 2024
- Select in the video player controls bar to choose subtitle language. Subtitles available in English, French, German, Spanish.
- Downloads including slides are available for this activity in the Toolkit
A fact sheet is available for this activity in the Toolkit
Learning Objectives
After watching this activity, participants should be better able to:
- Evaluate the latest data for approved and emerging tyrosine kinase inhibitors for chronic myeloid leukaemia in the first-line setting
- Discuss the data for approved and emerging tyrosine kinase inhibitors for chronic myeloid leukaemia in the second- and later-line settings
- Summarize the potential impact of emerging findings from clinical trials and real-world studies on current and future clinical practice in chronic myeloid leukaemia
Overview
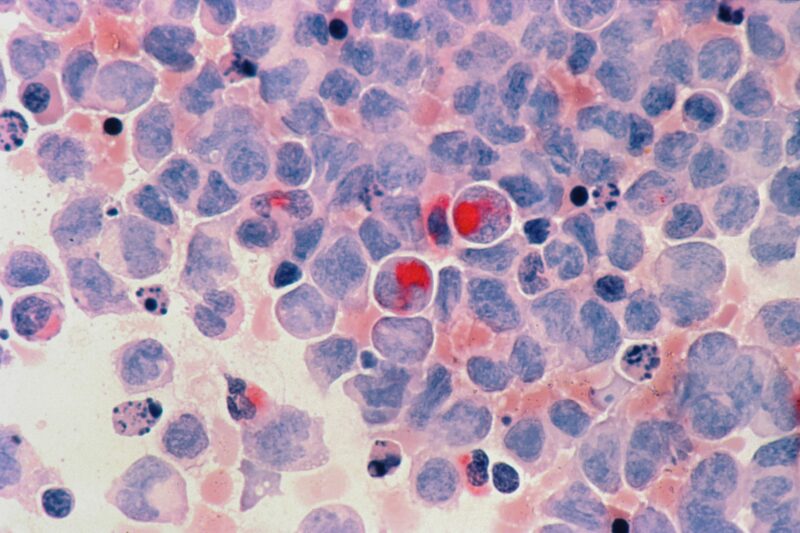
In this activity, an expert in chronic myeloid leukaemia (CML) reviews important data on approved and emerging agents for CML from key 2024 congresses, and discusses with two additional leading experts how this evidence may apply to clinical practice.
This activity was filmed following the ESH-iCMLf annual conference (Prague, Czech Republic, 27–29 September 2024) and is based on data from this meeting plus ASCO 2024 and EHA 2024.
This activity is provided by touchIME.
Target Audience
This activity has been designed to meet the educational needs of haematologists, including haemato-oncologists, and oncologists involved in the management of chronic myeloid leukemia (CML).
Faculty
Prof. Gianantonio Rosti discloses: Advisory board/panel fees from Incyte, Novartis and Sun Pharma. Grants/research support and speaker’s bureau fees from Incyte, Novartis, Pfizer and Sun Pharma.
Dr Carla Boquimpani discloses: Advisory board/panel fees from Janssen and Novartis. Speaker’s bureau fees from Janssen, Novartis, Pfizer and Pint Pharma.
Prof. Dr. med. Susanne Saussele discloses: Advisory board/panel fees from Incyte, Novartis, Pfizer and Roche. Grants/research support from BMS, Incyte and Novartis.
Touch Medical Contributors
Sola Neunie and Johanna Barry have no financial interests/relationships or affiliations in relation to this activity.
Date of original release: 28 October 2024. Date credits expire: 28 April 2026.
Time to Complete: 50 minutes
To obtain the credit(s) from this activity, please complete this post-activity test.
Claim CreditCourse Modules
- Select in the video player controls bar to choose subtitle language. Subtitles available in English, French, German, Spanish.
- Downloads including slides are available for this activity in the Toolkit
A fact sheet is available for this activity in the Toolkit
You may also be interested in...

REGISTER NOW FOR FREE ACCESS TO
- 1000+ topical and insightful peer-reviewed journal articles
- 100+ hours of bite-sized congress highlights
- 10 major therapy areas packed with the latest scientific advances
- 150+ specialties offering learn-on-the-go medical education
- + Concise email updates and newsletters so you never miss out

Log into your Touch Account
Earn and track your CME credits on the go, save articles for later, and follow the latest congress coverage.
Sign up with an Email
Or use a .
This Functionality is for
Members Only
Explore the latest in medical education and stay current in your field. Create a free account to track your learning.